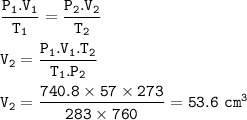The volume of gas when it is dry at S.T.P : 53.6 cm³
Further explanation
Given
P tot=750 mmHg
V₁=57 cm³
T₁=10 °C = 283 K
P₂ = 760 mmHg(STP)
T₂ = 273 K(STP)
Required
P dry gas at STP
Solution
Dalton's Law
P tot = P H₂O + P gas
P gas = 750 mmHg - 9.2 mmHg
P gas = 740.8 mmHg = P₁
Combined gas Law