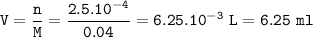The molarity = 0.04 M
The volume = 6.25 ml
Further explanation
Given
25cm³ of 0.1 M H₂SO₄
1.06g Na₂CO₃ in 250cm³ of solution
Required
the molarity and the volume of Na₂CO₃
Solution
Molarity of Na₂CO₃ :
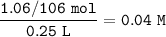
Reaction
H₂SO₄ + Na₂CO₃ ⇒Na₂SO₄ + H₂O + CO₂
mol H₂SO₄ = 25 x 0.1 = 0.25 mlmol= 2.5 x 10⁻⁴ moles of Na₂CO₃
The volume of solution used :