Answer:
52 mL
Step-by-step explanation:
Since the pressure of the gas remains constant, we can use the following gas law:
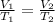
where:
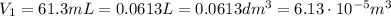 is the initial volume
is the initial volume
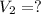 is the final volume
is the final volume
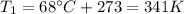 is the initial temperature
is the initial temperature
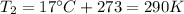 is the final temperature
is the final temperature
Substituting numbers and re-arranging the equation, we find the final volume of the gas:
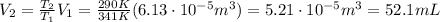
So the correct answer is
1. 52 mL