Answer:
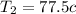
Step-by-step explanation:
From the question we are told that
Temp of first bolts

Temp of 2nd bolt
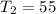
Generally the equation showing the relationship between heat & temperature is given by
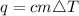
Generally heat released by the iron bolt = heat gained by the iron bolt
Generally solving mathematically
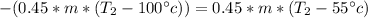
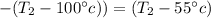
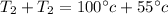
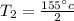
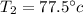
Therefore
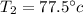 is the final temperature inside the container
is the final temperature inside the container