Answer: Option (C) is the correct answer.
Step-by-step explanation:
An element or substance whose oxidation state increases in a chemical reaction is known as reducing agent as the element itself gets oxidized.
On the other hand, an element or substance whose oxidation state decreases in a chemical reaction is known as an oxidizing agent as the element itself gets reduced.
For example,
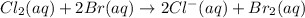
Oxidation-half reaction : 2Br^{-}(aq) \rightarrow Br_{2} + 1e^{-}[/tex]
Reduction-half reaction :
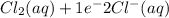
Hence, we can conclude that in this reaction
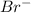 is the reducing agent.
is the reducing agent.