Answer:
The enthalpy of the overall chemical equation is -304.1 kJ
Step-by-step explanation:
Enthalpy is an additive property. The overall chemical equation can be obtained by summing up all three elementary steps. So enthalpy of overall reaction is summation of enthalpy changes in each steps.
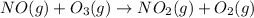
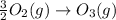
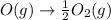
----------------------------------------------------------------------------------------
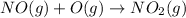
Enthalpy of overall reaction =
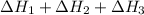
= (-198.9+142.3-247.5) kJ
= -304.1 kJ