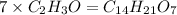Answer:The empirical formula is
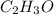 and molecular formula is
and molecular formula is
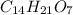
Solution : Given,
If percentage are given then we are taking total mass is 100 grams.
So, the mass of each element is equal to the percentage given.
Mass of C = 55.80 g
Mass of H = 7.04 g
Mass of O = 37.16 g
Step 1 : convert given masses into moles.
![Moles of C =[tex] \frac{\text{ given mass of C}}{\text{ molar mass of C}}= (55.80g)/(12g/mole)=4.65moles](https://img.qammunity.org/2020/formulas/chemistry/middle-school/eyld2shcws10fpuf4i4uqwwcpb8z1g2bms.png)
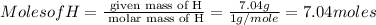
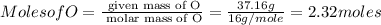
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For C =
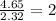
For H =
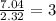
For O =
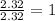
The ratio of C : H : O= 2:3:1
Hence the empirical formula is
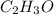
The empirical weight of
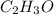 = 2(12)+3(1) +1(16)= 43g.
= 2(12)+3(1) +1(16)= 43g.
The molecular weight = 301.35 g/mole
Now we have to calculate the molecular formula.
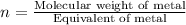
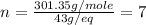
Thus molecular formula will be