Answer:
The mass of ammonium nitrite decomposed is 12.54 grams.
Step-by-step explanation:
Pressure of the nitrogen gas = P = 1 atm
Volume of the nitrogen gas = V = 4.40 L
Temperature of the nitrogen gas = T = 273.15 K
Moles of nitrogen gas = n
PV = nRT (ideal gas equation)
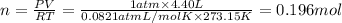
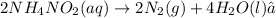
According to reaction , 2 mole of nitrogen is obtained from 2 mole of ammonium nitrite.
Then 0.196 moles of nitrogen will will be obtained from:
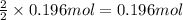 ammonium nitrite.
ammonium nitrite.
Mass of 0.196 moles of ammonium nitrite:
= 0.196 mol × 64 g/mol = 12.54 g
The mass of ammonium nitrite decomposed is 12.54 grams.