Answer:
The mass of calcium carbide is 1,392 grams.
Step-by-step explanation:
Pressure of the ethyne gas = P = 1.25 atm
Volume of the ethyne gas = V = 550 L
Temperature of the ethyne gas = T = 385 K
Moles of ethyne gas = n
PV = nRT (ideal gas equation)
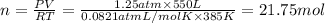
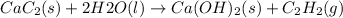
According to reaction , 1 mole of ethyne is obtained from 1 mole of calcium carbide.
Then 21.75 moles of ethyne will be obtained from:
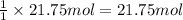 calcium carbide.
calcium carbide.
Mass of 21.75 moles of calcium carbide:
= 21.75 mol × 64 g/mol = 1,392 g
The mass of calcium carbide is 1,392 grams.