The standard ambient temperature and pressure are
Temperature =298 K
Pressure = 1atm
The density of gas is 1.5328 g/L
density = mass of gas per unit volume
the ideal gas equation is
PV = nRT
P = pressure = 1 atm
V = volume
n = moles
R= gas constant = 0.0821 Latm/mol K
T = 298 K
moles = mass / molar mass
so we can write
n/V = density / molar mass
Putting values
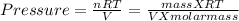
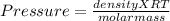
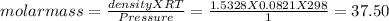
Thus molar mass of gas is 37.50g/mol