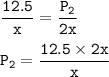The new pressure will be doubled
Further explanation
Given
A sample of ozone (O₃) at x K and 12.5 Pa. (T₁= x K, P₁=12.5 Pa)
the temperature is doubled to 2x K (T₂=2x)
Required
the new pressure
Solution
Gay Lussac's Law
When the volume is not changed, the gas pressure is proportional to its absolute temperature
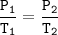
Input the value :