Answer:
Shifts to products.
Step-by-step explanation:
the reaction is
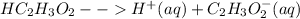
If we add base (NaOH) to it, the base will react with the hydrogen ions decreasing the concentration of products. This will shift the equilibrium towards products.
As per Le-Chatelier's principle, if we apply any change to an equilibrium system, the system shifts in the direction where it can nullify the effect of stress applied. As we are decreasing the concentration of products the system will shift towards product to increase the concentration further.