Explanation :
(1) Sodium hydroxide and calcium bromide
The balanced chemical reaction will be,
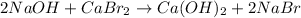
This reaction is a double displacement reaction in which a positive cation and a negative anion of two reactants exchange their places to form two new products.
(2) Magnesium oxide
The balanced chemical reaction will be,
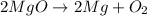
This reaction is a decomposition reaction in which a larger reactant molecule decomposes into two or more smaller product molecules.
(3) Aluminum and iron(II)nitrate
The balanced chemical reaction will be,
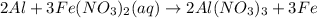
This reaction is a single displacement reaction in which the most reactive element displaces the least reactive element.
(4) Sodium and Chlorine
The balanced chemical reaction will be,
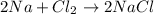
This reaction is a combination reaction in which the two or more reactant combine to form a new product.