Answer:
The average kinetic energy of their particles is the same
Step-by-step explanation:
The temperature of an object/substance is directly related to the average kinetic energy of the particles in the object, according to:
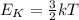
where
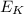 is the average kinetic energy of the particles
is the average kinetic energy of the particles
k is the Boltzmann's constant
T is the temperature of the object
Since k is the constant value, from the equation we see that if two objects of same nature have same temperature T, then the average kinetic energy of their particles, Ek, is the same.