Answer: Option (D) is the correct answer.
Step-by-step explanation:
It is known that
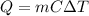
where Q = heat
m = heat
C = specific heat
 = change in temperature
= change in temperature
Therefore, we can see that heat is proportional to mass. Hence, more is the mass of a substance more it contains or requires heat.
Therefore, when a large block of ice is added to a hot cup of water then it won't melt the ice because mass of water is less. Therefore, heat released or given out by it will be less.
Whereas lake is able to dissolve the large block of ice because lake has large mass and thus it will have more heat.
Therefore, we can conclude that the lake contains more mass and more heat than the cup of hot water.