Answer:
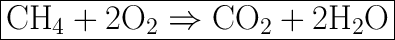
![\rule[225]{225}{2}](https://img.qammunity.org/2020/formulas/english/high-school/uddwtr8rtj6rh4i3za37iy1kf4x6nmf1o7.png)
Step-by-step explanation:
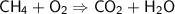
Balancing the Hydrogen atoms on the right side,
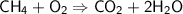
Balancing the Oxygen atoms on the left side,
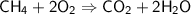
In a balanced equation, the product side and the reactant side must have the same mass of matter, since matter cannot be destroyed or created according to the law.
![\rule[225]{225}{2}](https://img.qammunity.org/2020/formulas/english/high-school/uddwtr8rtj6rh4i3za37iy1kf4x6nmf1o7.png)