Answer:
The correct answer is: It is a combustion reaction, because oxygen reacts with a substance.
Step-by-step explanation:
Combustion reaction defined as chemical reaction which hydrocarbon reacts with oxygen molecule to give water, carbon dioxide and heat as a product.
Here in the give reaction methane is undergoing combustion reaction in presence of oxygen to give water and carbon dioxide.The balanced chemical reaction is given as:
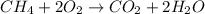
Whereas in synthesis reaction is type of chemical reaction in which two more reactant reacts together to form a single product.
A + D +C → D