Answer:
5.6 ounces of 30% H₂O₂ solution should be added.
Explanation:
Let x ml of 30% H₂O₂ solution should be added with 10% H₂O₂ solution to get 3% H₂O₂ solution.
Volume of 3% H₂O₂ solution = 16 ounces
Volume of 10% H₂O₂ solution after mixing 30% H₂O₂ solution = (x + 16) ounces
Now equation will be
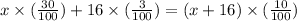
0.3x + 0.48 = 0.10(x + 16)
0.3x + 0.48 = 0.10x + 1.6
0.3x = 0.10x + 1.6 - 0.48
0.3x - 0.10x = 1.6 - 0.48
0.20x = 1.12
x =
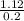
= 5.6 ounces