Answer : The oxidation number of Ti in
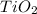 is, (+4)
is, (+4)
Explanation :
Oxidation number : It is also known as the oxidation state. It is defined as the number of electrons gained or lost by the atom of an element in the compound is assigned by the number.
The given molecule is,
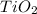
Let the oxidation number of Ti be 'x' and the oxidation state of oxygen is, (-2)
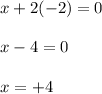
Hence, the oxidation number of Ti in
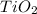 is, (+4)
is, (+4)