Answer: Chlorine will become a negative ion.
Step-by-step explanation:
A negative ion is formed when an element has more electrons than its ground state configuration. This negative ion is known as an anion.
These ions are most favorably formed by the elements present in Group 17 because these elements only require 1 electron to attain a stable electronic configuration.
Electronic configuration of Group 17 elements:
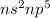
Carbon is a group 14 element and hence, will not easily form a negative ion.
Nitrogen is a group 15 element and hence, will not easily form a negative ion.
Aluminium is a group 13 element and it forms a positive ion.
Chlorine is a group 17 element and it most likely forms a negative ion when forming a chemical bond.
Hence, the correct answer is Chlorine.