Answer: The correct answer is temperature.
Step-by-step explanation:
Average kinetic energy is defined as the average of kinetic energies of all the particles in a system. It is a measure of temperature.
Mathematically,
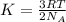
where,
K = average kinetic energy
R = Gas constant
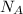 = Avogadro's number
= Avogadro's number
T = temperature of the system
As, the temperature of the system increases, the average kinetic energy of the particles also increases and vice-versa.
Thus, the correct answer is temperature.