Answer:- (1) 0.075 M, (2) 0.00768M and (3) 0.106M.
Solution:- (1) Molarity is moles of solute per liter of solution. From given volume and molarity we can calculate the moles of NaOH.
We convert mL to L and multiply by molarity to get the moles.
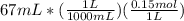
= 0.01005 mol NaOH
NaOH and HCl react in 1:1 mol ratio which is clear from the below equation:-
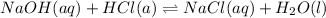
As they react in 1:1 mol ratio, the moles of HCl will also be 0.01005. Volume of HCl is given. We convert the mL to L and divide the moles by it to get the molarity of HCl.
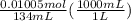
= 0.075 M
So, the molarity of HCl solution is 0.075M.
(2) This one is also similar to the previous one. Only the mol ratio will be different as per the equation:
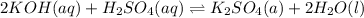
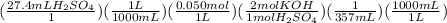
= 0.00768M KOH
So, the concentration of KOH(or if it is even NaOH) is 0.00768 M.
(3) This one is exactly similar to the second one. The balanced equation is:
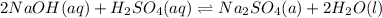
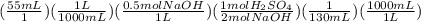
= 0.106M

So, the molarity of the sulfuric acid is 0.106 M.
(4) In acid base titration, the end point is the point at which the indicator changes into color where as the equivalence point is the point at which the moles of acid and base are in their stoichiometric ratio.
For example if we have 0.2 moles of sulfuric acid then there must be 0.4 moles of NaOH at equivalence point since they react in 1:2 mol ratio.