Answer: Carbon-carbon double bond is stronger and shorter than the single bond.
Step-by-step explanation:
It is given that carbon-carbon double bond has greater energy than the carbon-carbon single bond.
Bond energy is directly proportional to the bond strength, which means that the double bond will have greater strength than single bond and triple bond has the greatest strength of all the bonds.
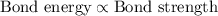
Bond energy is inversely proportional to the bond length of the carbon-carbon bond. This means that more is the bond energy, shorter will be the bond and vice-versa.
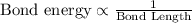
Hence, carbon-carbon double bond is stronger and shorter than the single bond.