The given equation is is
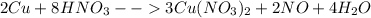
this is not balanced
The balanced equation will be:
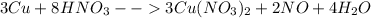
Now as per it if we are using eight moles of HNO3 we are getting two moles of NO
so for each mole of NO we have to take four moles of HNO3
Hence the mole ratio of NO produced to HNO3 reacted will be = 1:4