Answer : The correct option is, (A) forms colorful compounds.
Explanation :
As we know that nickel (Ni) is transition metal with atomic number 28 and it is belong to the 3-d series in a periodic table.
The electronic configuration of 'Ni' is :
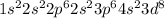
It is a transition elements that has ability to shows variable oxidation state and due to variable oxidation state they forms colorful compounds.
Hence, the correct option is, (A) forms colorful compounds.