Answer: The mass of potassium bromide present in 41.2 mL of solution will be 3.522 grams.
Step-by-step explanation:
We are given that KBr is present in 8.3% KBr solution, which means that 8.3 grams of potassium bromide is present in 100 gram of the solution.
To calculate the volume of KBr, we use the formula:

Mass of the solution = 100 grams
Density of KBr solution = 1.03g/mL
Volume of the solution = ? mL
Putting values in above equation, we get:
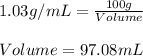
Now, to calculate the mass of KBr in 41.2mL of the solution, we use unitary method.
In 97.08 mL of solution, mass of KBr present is 8.3 grams.
So, 41.2 mL of solution will contain =
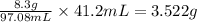 of KBr.
of KBr.
Hence, the mass of potassium bromide present in 41.2 mL of solution will be 3.522 grams.