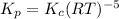Answer: The equation which is wrong is
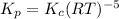
Step-by-step explanation:
For the given reaction:
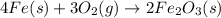
The expression for
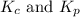 is given by:
is given by:
![K_c=(1)/([O_2]^3)](https://img.qammunity.org/2020/formulas/chemistry/college/t0nzgzjjmf7gflbnx6sk94ofs55d6t2xzu.png)
![K_p=(1)/([O_2]^3)](https://img.qammunity.org/2020/formulas/chemistry/college/j2c7fh1pgwwrl9lioehdgq8mkb66whglzn.png)
The concentration of solids are taken to be 1, only concentration of gases and liquid states are taken. The pressure of only gases are taken.
Relationship between
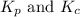 is given by the expression:
is given by the expression:
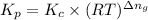
where,
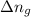 = number of moles of gaseous products - number of moles of gaseous reactants
= number of moles of gaseous products - number of moles of gaseous reactants
R = gas constant
T= temperature
For the above reaction,
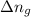 = number of moles of gaseous products - number of moles of gaseous reactants = 0 - 3 = -3
= number of moles of gaseous products - number of moles of gaseous reactants = 0 - 3 = -3
Hence, the expression for
 is:
is:
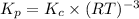
Therefore, the equation which is wrong is