Answer: The correct answer is constant temperature and pressure.
Step-by-step explanation:
From a balanced equation, we can easily judge the mole ratio of the substances present. And from the mole ratio, we can calculate the number of moles and from moles, we can calculate the volume and then volume ratio of the substances.
Moles and volume are related to each other by the law given by Charles'.
This law states that volume is directly proportional to the moles at constant pressure and temperature.
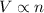 (At constant volume and number of moles)
(At constant volume and number of moles)
or,
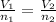
Hence, the correct answer is constant temperature and pressure.