Answer:
add N2O4
Step-by-step explanation:
According to Le Chatelier's principle when a chemical reaction at equilibrium is subject to changes in temperature, pressure or concentration then the equilibrium will shift in a direction so as to undo the effect of the induced change.
The given reaction is the decomposition of N2O4 into NO2
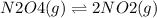
In order to shift the equilibrium to the right i.e towards the product, add N2O4. In accordance with Le Chatelier's principle, adding N2O4 will shift the equilibrium in a direction which would facililate the consumption of N2O4 i.e. towards the right thereby favoring the formation of NO2.