Answer:
Step-by-step explanation:
Percent composition tells us the mass percentage of an element in a compound. It can be found by dividing the mass of the element by the mass of the compound, then multiplying by 100.
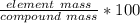
1. Compound Mass
The compound given is HNO₃
- Hydrogen (H) and Nitrogen (N) have no subscript, implying there is 1 atom of each element.
- Oxygen (O) has a subscript of 3, so there are 3 atoms.
Use the Periodic Table to find the masses of each element.
- Hydrogen: 1.008 u
- Nitrogen: 14.007 u
- Oxygen: 15.999 u
Next, multiply each element's mass by the number of atoms, then add them together.
- (1.008 u) + (14.007 u) + (3 * 15.999 u)
- 63.012 u
2. Percent Composition
Now we know that HNO₃'s mass is 63.012 u. This is the compound mass.
We want to find the percent of hydrogen and its mass is 1.008 u. This is the element mass.
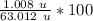
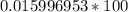
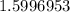
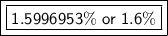
This can be rounded to the nearest tenth. The 9 in the hundredth place tells us to round the 5 to a 6.
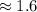
The percent of hydrogen in HNO₃ is 1.5996953% or about 1.6%