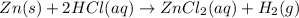Answer: The atoms of every element on both the sides of the reaction must be same.
Step-by-step explanation:
We are given a chemical equation and we need to balance it. Every equation follows Law of Conservation of mass.
This law states that in a chemical reaction, the number of atoms of each element must be same on both the sides of the equation.
For the given chemical equation:
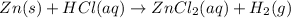
On reactant side:
Number of zinc atoms = 1
Number of hydrogen atoms = 1
Number of chlorine atoms = 1
On product side:
Number of zinc atoms = 1
Number of hydrogen atoms = 2
Number of chlorine atoms = 2
As, the number of hydrogen and chlorine atoms on reactant and product side is not same, so we need to add a stoichiometric coefficient in-front of HCl on the reactant side. The balanced chemical equation becomes: