Answer:
Three hydrogen atoms to form PH₃.
Step-by-step explanation:
Hello!
In this case, since the elements belonging to the nitrogen family (N, P, As, Sb and Bi) show five valence electrons, because there are five electrons at their outer shell, it is clear that if phosphorous bonds with hydrogen, it is going to require the same amount of oxygen atoms (3) because elements having five valence electrons need 3 bonds in order to attain the octet (5+3=8).
Therefore the compound would be:
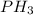
Which is phosphine.
Best regards!