Hello!
The answer is A. the principal energy level
Why?
The principal energy level or principal quantum number n, tell us about the position of an determined electron in the energy levels relative to the greater average distance of an electron from the nucleus, the larger the value of n and the higher its energy.
The principal energy levels contains n sublevels,
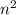 orbitals and
orbitals and
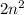 electrons.
electrons.
For example, the number 1, has one orbital which is contained in a energy sublevel, its called s orbital, and it's just an orbital with 2 electrons.
Have a nice day!