Here we can say that number of moles as well as pressure will remain same for the balloon
so here we can say by ideal gas equation
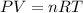
so here for constant pressure and constant number of moles we have
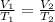

now by rearranging the equation we have
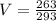
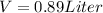
so final volume will be 0.89 liter