Answer: 34.65 N towards charge 8 μC.
Step-by-step explanation:
The electrostatic force between two charges is given by:
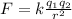
where, k is the Coulomb constant = 8.9875 × 10⁹ N.m²/C²
q₁ and q₂ are the two charges separated by distance r.
The distance between charges 8 μC and -7 μC is r = 2 cm -(-10 cm) = 12 cm = 0.12 m
The force between these charges is:
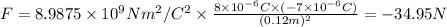
Negative sign implies it is an attractive force.
The distance between -3 μC charge and -7 μC charge is r' = 10 cm -2 cm = 8 cm = 0.8 m.
The electrostatic force between these charges is:
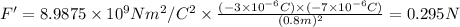
It is a repulsive force.
Net force on the -7 μC charge is:
Fn = F + F'
we can add them directly as they are acting in one direction along the x-axis.
Fn = -34.95 N + 0.295 = -34.65 N
Thus, the net force is attractive in nature and it is towards charge 8 μC.