Answer:
The pH of the resulting solution will be 7.
Step-by-step explanation:
Sodium chloride is a salt that is formed from the reaction of sodium hydroxide (a strong base) and hydrochloric acid ( a strong acid), as shown below.
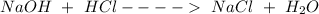
Generally, when strong acids react with strong bases, neutral salts and water are formed. Sodium Chloride (NaCl) is an example of such neutral salts formed.
Since sodium chloride (NaCl) is neutral, its concentration will not alter the pH of the water and the pH of the resulting solution will be 7.