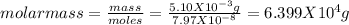The molecular weight of hemoglobin can be calculated using osmotic pressure
Osmotic pressure is a colligative property and it depends on molarity as
πV = nRT
where
π = osmotic pressure
V = volume = 1mL = 0.001 L
n = moles
R = gas constant = 0.0821 L atm / mol K
T = temperature = 25°C = 25 + 273 K = 298 K
Putting values we will get value of moles
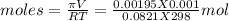
we know that
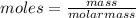
Therefore