Answer:

Step-by-step explanation:
We can use the specific heat formula, which is:
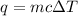
Where q is the energy, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
We know the mass is 250 grams. The specific heat capacity of water is 4.18 joules per gram degree Celsius. Let's find the change in temperature.
- ΔT = final temperature - initial temperature
Now we know all the values:
Substitute the known values into the formula.
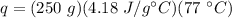
Multiply the first 2 numbers together. The grams will cancel out.
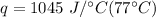
Multiply again. This time the degrees Celsius will cancel out.
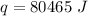
The energy needed is 80, 465 Joules.