Answer: Table salt
Step-by-step explanation: Sucrose
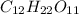 is a covalent compound which is formed by sharing of electrons and thus form a weak bond.
is a covalent compound which is formed by sharing of electrons and thus form a weak bond.
Table salt
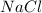 is a ionic compound formed by transfer of electrons and thus a strong bond is formed due to strong coloumbic forces.Thus large amount of energy is required to break the bonds and they have high melting points.
is a ionic compound formed by transfer of electrons and thus a strong bond is formed due to strong coloumbic forces.Thus large amount of energy is required to break the bonds and they have high melting points.
Glucose
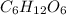 is a covalent compound which is formed by sharing of electrons and thus form a weak bond.
is a covalent compound which is formed by sharing of electrons and thus form a weak bond.
Butter is also a covalent compound which is formed by sharing of electrons and thus form a weak bond.