Answer: D. None of these will increase the half life.
Step-by-step explanation:
Half life is the amount of time taken to decay to half of its original value. The half life is related to rate constant by the following relation for a first order reaction:
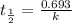
As half life is independent of concentration , temperature and pressure, none of these factors would affect the half life.
Thus in the reaction,
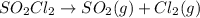 following first order kinetics, none of the factors will increase the half life.
following first order kinetics, none of the factors will increase the half life.