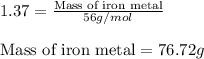Answer:
For 1: The correct answer is 407.97 grams.
For 2: The correct answer is 76.72 grams.
Step-by-step explanation:
We are given the molarity of HCl, to find the moles of HCl, we use the formula:
![Molarity=\frac{\text{Moles}{\text{Volume}}]()
We are given:
Molarity = 1.6 M
Volume = 7.8 L
Putting values in above equation, we get:
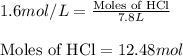
For the given reaction:
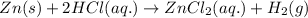
By Stoichiometry of the reaction:
2 moles of HCl reacts with 1 mole of zinc metal.
So, 12.48 moles of HCl react with =
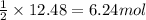 of Zinc metal.
of Zinc metal.
To calculate the mass of zinc metal, we use the formula:
 .....(1)
.....(1)
Molar mass of Zinc metal = 65.38 g/mol
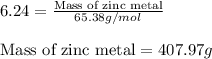
We are given that 298 grams of 83.1 % by mass of iron (II) nitrate solution are present.
So, mass of Iron (II) nitrate solution will be =
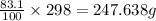
Molar mass Iron (II) nitrate = 180 g/mol
Putting values in equation 1, we get:
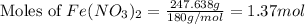
For the following reaction:
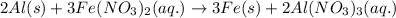
By Stoichiometry of the reaction:
3 moles of
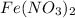 produces 3 moles of iron metal.
produces 3 moles of iron metal.
So, 1.37 moles of
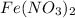 will produce =
will produce =
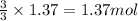 of iron metal.
of iron metal.
To calculate the mass of zinc metal, we equation 1: