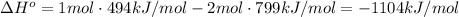Answer:
-1104 kJ/mol
Step-by-step explanation:
The change in the enthalpy of a reaction is equal to the difference between: the sum of the enthalpy changes of the bonds broken and the sum of the enthalpy changes of the bonds formed.
The bonds broken correspond to the cleavage of bonds of the reactants, the bonds formed correspond to the bonds of the products:
- we only break oxygen O=O bond, since carbon is not bonded to anything;
- we form two C=O bonds in carbon dioxide.
Therefore, the enthalpy change is calculated by: