The relation between rate constants at different temperatures, temperature and activation energy is known as Arrhenius equation
Arrhenius equation
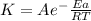
For two temperatures
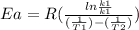
Where
Ea = ? = activation energy
k1 = 3.36 × 10⁴
T1=344 k
k2=7.69
T2=219K
R= gas constant = 8.314 J /molK
Putting values
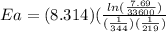
Ea = (-69.69)/(-0.00166) = 41981.93 J/mol
Or
Activation energy is 42.0 kJ /mol