- Mg(OH)₂ (s) + H₂SO₄ → MgSO₄ (s) + 2 H₂O (l).
- HCl (aq) + KOH (l) → KCl (aq) + H₂O (l).
- NH₃ (aq) + HCl (aq) → NH₄Cl (aq).
Step-by-step explanation
There are three acid-base theories: Arrhenius, Bronsted-Lowry, and Lewis. Among the three, only the Bronsted-Lowry theory defines acids and bases based on the transfer of protons H⁺.
- A Bronsted-Lowry acid is a proton donor;
- A Bronsted-Lowry base is a proton acceptor.
Mg(OH)₂ (s) + H₂SO₄ → MgSO₄ (s) + 2 H₂O (l)
- Mg(OH)₂ acts as a base and accepts protons H⁺.
- H₂SO₄ acts as an acid and loses protons H⁺.
Fe(NO₃)₃ (aq) + 3 KOH (aq) → Fe(OH)₃ (s) + 3 KNO₃ (aq)
- There's a transfer of OH⁻ and NO₃⁻ but not protons H⁺.
Zn (s) + Cu(NO₃)₂ (aq) → Zn(NO₃)₂ (aq) + Cu(s)
- Zn loses electrons to Cu²⁺ in Cu(NO₃)₂.
- There's no transfer of protons H⁺.
Mg (s) + Cu(NO₃)₂ (aq) → Mg(NO₃)₂ + Cu (s)
- Mg loses electrons to Cu²⁺ in Cu(NO₃)₂.
- There's no transfer of protons H⁺.
HCl (aq) + KOH (aq) → KCl (aq) + H₂O (l)
- HCl acts as an acid and loses protons H⁺.
- KOH acts as a base and gains protons H⁺.
NH₃ (aq) + HCl (aq) → NH₄Cl (aq)
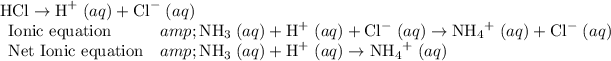
- HCl acts as an acid and loses protons H⁺.
- NH₃ acts as a base and gains protons H⁺.