Answer : The number of moles of aluminium oxide produced from 0.35 moles of oxygen are, 0.233 moles.
Solution : Given,
Moles of oxygen = 0.35 moles
The balanced chemical reaction will be,
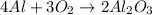
From the balanced chemical equation, we conclude that
As, 3 moles of oxygen react to give 2 moles of aluminium oxide
So, 0.35 moles of oxygen react to give
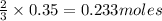 of aluminium oxide
of aluminium oxide
Therefore, the number of moles of aluminium oxide produced from 0.35 moles of oxygen are, 0.233 moles.