Answer: a) Tl (Thallium)
b)
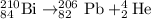
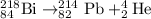
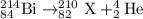
Step-by-step explanation:
Alpha decay : When a larger nuclei decays into smaller nuclei by releasing alpha particle. In this process, the mass number and atomic number is reduced by 4 and 2 units respectively.
General representation of an element is given as:
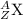
where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
Total mass on reactant side = total mass on product side
214= A + 4 , A = 210
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
83= Z + 2, Z = 81
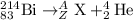
b)
1.
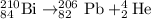
Total mass on reactant side = total mass on product side
210= A + 4 , A = 206
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
84= Z + 2, Z = 82
2.
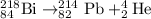
Total mass on reactant side = total mass on product side
218= A + 4 , A = 214
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
84= Z + 2, Z = 82
3.
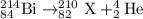
Total mass on reactant side = total mass on product side
214= A + 4 , A = 210
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
84= Z + 2, Z = 82