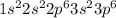Answer : The electronic configuration of argon (Ar) is,
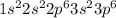
Explanation :
Element is, Argon (Ar)
Atomic number of argon = 18
The number of electrons = 18
As we know that the number of electrons is equal to the number of protons and the number of proton is equal to the atomic number.
Electronic configuration : It is defined as the arrangement or distribution of electron of atom in a atomic orbital.
Hence, the electronic configuration of argon is,