Answer:
The dryer evaporate 200 - 24.74 kg of water per hour
To remove 1kg of water it need 3000 K J
So to remove, 175.26 Kg
it need 5.257x
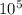 KJ of heat per hour.
KJ of heat per hour.
Step-by-step explanation:
In one hour, the amount of sugar entering = 1000 kg
w.b moisture content is defined as,
weight of water / weight of water + weight of dry
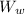 /
/
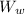 +
+
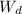 x 100
x 100
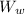 +
+
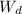 = 1000 kg when entering
= 1000 kg when entering
it has 20% moisture content when entering
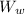 = 0.2 x 1000 = 200 kg
= 0.2 x 1000 = 200 kg
when leaving it has 3% moisture content then weight of dry material
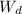 = 1000 - 200 = 800 Kg
= 1000 - 200 = 800 Kg
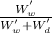 = 0.03
= 0.03
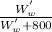 = 0.03
= 0.03
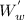 = 0.03 x
= 0.03 x
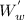 + 0.03 x 800
+ 0.03 x 800
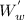 = 24.74 kg
= 24.74 kg
When leaving the dryer, the crystals has total weight of the water = 24.74 kg per hour.
The dryer evaporate 200 - 24.74 kg of water per hour
To remove 1kg of water it need 3000 K J
So to remove, 175.26 Kg
it need 5.257x
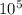 KJ of heat per hour.
KJ of heat per hour.