The gas laws describe and predict the behavior of gases with an explanation and experimental data
So the given statement is False.
2) The volume of gas can be calculated based on Avagadro's law
It states that the volume of a gas is directly proportional or varies with the moles of the gas. Higher the moles more the volume, condition is the pressure and temperature are constants in the two conditions
Thus as here the pressure and temperature of nitrogen gas is kept constant
V α moles
or
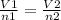
Where
V1 = 6 l
n1 = 0.50 mol
V2 = ?
n2 = 0.75 mol
On putting values
V2 = 6 X 0.75 / 0.5 = 9 L
so resulting volume of the gas will be 9L