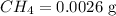Answer:
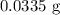
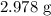
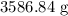
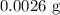
Step-by-step explanation:
Mass in grams is given by
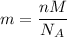
where
n = Number of molecules
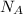 = Avogadro's number =
= Avogadro's number =
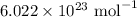
M = Molar mass of molecule
Molar mass of
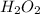 = 34.0147 g/mol
= 34.0147 g/mol
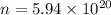
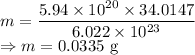
Mass of
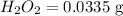
Molar mass of
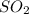 = 64.066 g/mol
= 64.066 g/mol
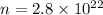
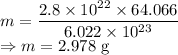
Mass of
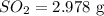
Molar mass of
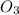 = 48 g/mol
= 48 g/mol
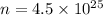

Mass of
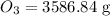
Molar mass of
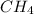 = 16.04 g/mol
= 16.04 g/mol
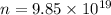
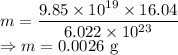
Mass of